There are several candidates talking about the high costs of drugs. The Republican front-runner, Donald Trump, has said in his town halls that we could save $300 billion a year by negotiating pricing through competitive bidding. Bernie says drug companies make outrageous profits and through a single payer system, he would dramatically lower costs. Hillary says there will be a monthly cap on what Americans will pay for drugs, and she will institute Medicare price controls, and end tax deductions for DTC.

-Bob Ehrlich
Mr. Trump has greatly exaggerated the potential savings. Medicare spent about $85 billion on prescription drugs through its Part D program in 2015. Total spending for drugs in the United States was $374 billion in 2014. Assuming price negotiation through Medicare can match Canadian prices, Medicare could save about 30% or $25-26 billion. Assuming private insurers would use these new Medicare prices to help negotiate harder, maybe they can squeeze another 10% out of drug makers. That would add another $28-30 billion.
Let’s say the public and private sector savings add to $53-56 billion annually. And for sake of argument let’s assume both Bernie and Hillary plans get the same. What happens to the drug industry losing $53-56 billion in revenue? About 85-90% of the lost revenue would be profit. That means about $45 billion. Total drug industry profits are in the $100 billion range.
What would happen to an industry that loses 45% of its annual profit? Massive layoffs in all areas of operations, relocation of jobs that remain to China and India, slashing of research budgets, and a focus on less risky drugs would occur. Bernie may think drug companies make outrageous profits but he has no experience how businesses work since he never had a regular private sector job. High risk industries need large incentives to take risks. Many drug companies fail in their research efforts and lose billions doing so.
Assuming Mr. Trump or Hillary succeed in price controls, they will turn the drug industry into a quasi government contractor. New drugs will be those that government agrees to subsidize. That means many smaller disease categories will be ignored as government will focus on the diseases that impact broad populations.
Expect drug marketing to disappear under a government dominated system. Medicare under its price negotiation authority would discourage, if not outright ban drug advertising. After all they do not want Americans to use expensive drugs. All of us in DTC will need to find other work.
There may be private innovators who still develop breakthrough drugs. It will be harder under reduced pricing and I suspect fewer small drug companies will get venture capital. Government will have an increased role in basic research and drug companies will be secondary. Nationalizing R&D sounds great to socialists, but how many new drugs came out of the Soviet Union?
An electorate hostile to drug companies may actually buy into the narrative that price controls work. Our total health expenditures are about $3 trillion in the United States. It would be shame to gut the vital drug industry for a $50 billion saving. Next time we see an Ebola outbreak, a Zika like virus, a new flu, or a drug resistant bacteria, we will want a vibrant drug industry to give us cures. While politically popular to rail against drug companies, the American people need to understand the unintended consequences from a punitive approach to drug profitability.




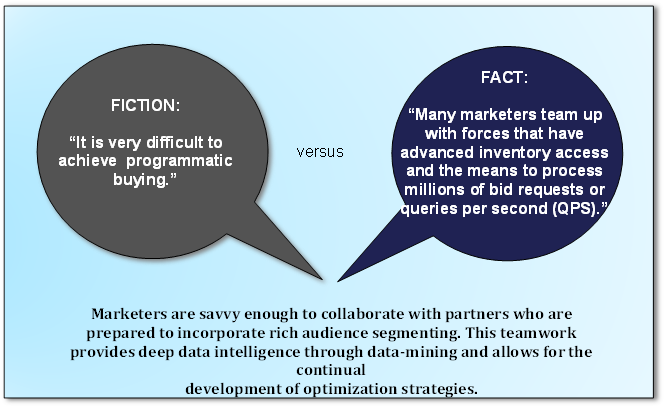
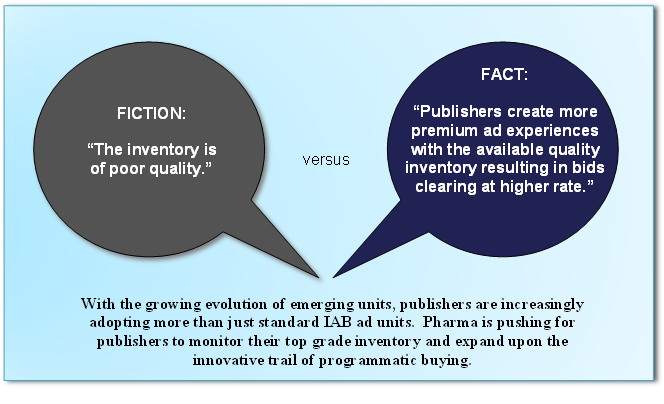
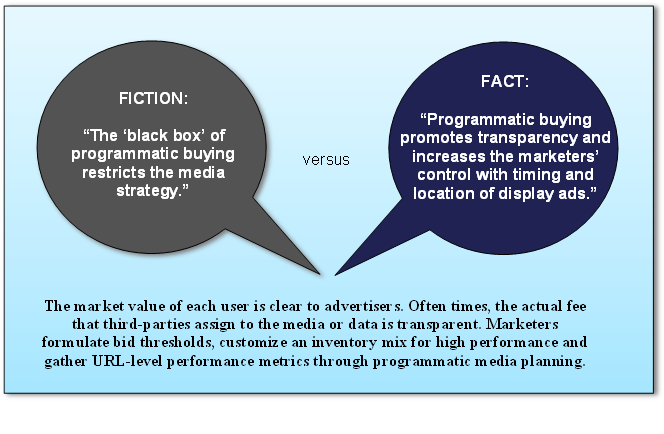
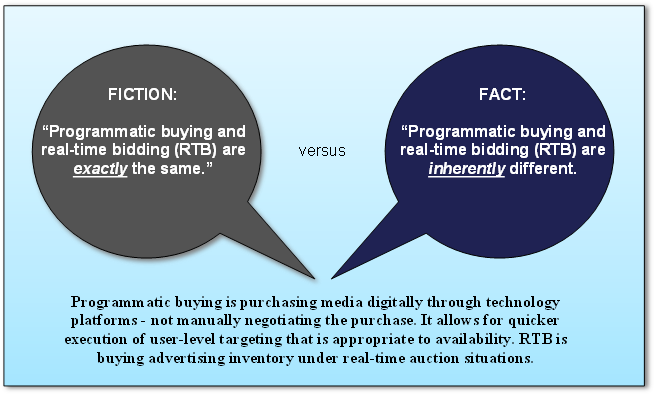
 Managing Global Frequency. Marketers effectively map users to all of their devices, match users across a myriad of platforms and determine frequency to an individual. They control their messaging and influence their bidding strategies to usher users into frequency where conversions emerge.
Managing Global Frequency. Marketers effectively map users to all of their devices, match users across a myriad of platforms and determine frequency to an individual. They control their messaging and influence their bidding strategies to usher users into frequency where conversions emerge.


