Should Pharma Site Metadata Play by the Same Rules as Paid Search Ads?
This is the second entry in the series about “promotional peccadilloes” available on Convergence Point Media’s Insights blog – topics that often trigger a perceived conflict between good regulatory compliance and effective marketing, and our perspectives on best practices to address them.
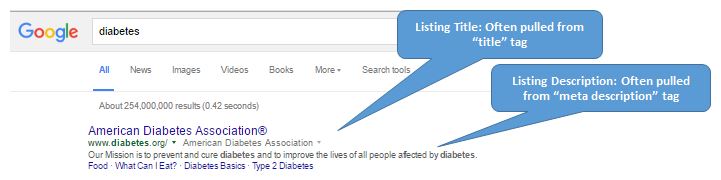
 The SERP (Search Engine Results Page) has become the primary gateway for patients seeking health information. As people research a condition or its treatment options, search results often serve as a brand’s first impression, playing a critical role in the split-second decision to click and engage, or move on to other results. While organic search results are controlled by proprietary search engine algorithms, site owners are responsible for the meta tags placed on their pages.
The SERP (Search Engine Results Page) has become the primary gateway for patients seeking health information. As people research a condition or its treatment options, search results often serve as a brand’s first impression, playing a critical role in the split-second decision to click and engage, or move on to other results. While organic search results are controlled by proprietary search engine algorithms, site owners are responsible for the meta tags placed on their pages.
A brand’s decisions about SEO meta tags can have very real implications on organic (non-paid) site traffic and engagement, so hitting the mark on both compliance and effectiveness is a significant competitive advantage. To be blunt, it’s easy to be compliant by erring on the side of extreme caution – but the concerns of both marketers and reviewers are best address holistically, giving rise to informed decision-making with sensible safeguards resulting in better digital outcomes. SEO metadata is a shining example of how this can play out.
A little refresher may help put this in perspective. Meta tags play an important role in a website’s discoverability via organic search results – or, as described by Google, a “way for webmasters to provide search engines with information about their sites”. They are strings of HTML code that provide descriptive information about the content of the web pages they are placed on, meant to help search engine’s automated crawlers accurately formulate relevant search results and appropriate rankings. Here, we will focus on the two types of meta tags found on web pages that are available to search engine algorithms in generating organic listings.
- Title Tag:
- Function: accurately and concisely describes the page’s thematic topic
- Appears in page source code as: <title>Page title here</title>
- Meta Description Tag:
- Function: provides a short description of the page’s content
- Appears in page source code as: <meta name=“description” content=”Page description here” />
While the organic listings users see on the SERP may include the provided meta tags, Google’s generation of organic results “is completely automated and takes into account both the content of a page as well as references to it that appear on the web.” Importantly, even if site owners omit meta descriptions altogether, the engines crawl the page content and other sources to determine what will be displayed in the search result. With respect to best practices for their intended use, Google states that “the goal of the snippet and title is to best represent and describe each result and explain how it relates to the user’s query. The more information you give us, the better your search result snippet can be.”
Since title and description tags are often pulled by the engine crawlers into the results users see on the SERP, this sets up what may be perceived by brands as a conflict between best practice website development and promotional compliance. The simple question we address today is “should branded pharma web sites restrict their meta data to conform to compliance standards used for paid search ads?”
State of the industry – Who’s doing what?
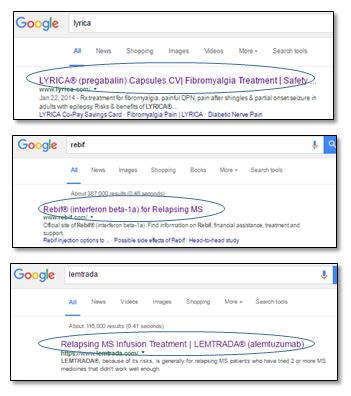
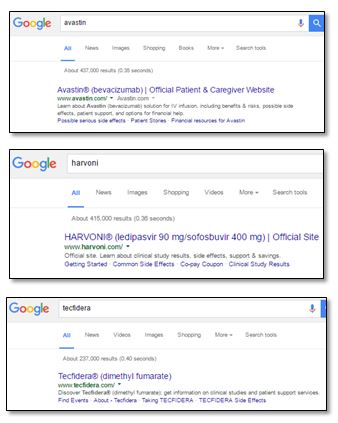
Within the Rx pharmaceutical industry, where promotional practices are often consistent across major manufacturers, brands appear split into two groups with respect to their approach to website title and description tags. One group conforms with the intended purpose of the metadata elements, using the available space to fully summarize the site or page content for the benefit of the search engine algorithms, typically including prominent naming of the disease state the product is approved to treat. Others, however, restrict meta tags on branded sites to conform with the “reminder and “reminder-like” ad format for compliant branded paid search ads (as defined by Dale Cooke on the Regulatory Rx Blog). These include the brand and generic names and may contain dosage form, quantitative ingredient statements and other descriptive information provided they make “no representation or suggestion” about the product use.
Examples of brands not restricting metadata:
Examples of brands restricting metadata (using “reminder” or “reminder-like” format):
Below we summarize key considerations with respect to both common approaches:
Reasons to restrict metadata:
- Applying the principles of the “reminder format” essentially treats meta data as equivalent to direct brand communications and increases the probability that an organic search result will be constructed by the search engine without implying a claim
- This is arguably the most conservative and risk-averse approach from a regulatory perspective as it removes fair balance from the equation altogether
Reasons not to restrict metadata:
- Irrespective of the presence of meta data, search engine algorithms ultimately determine what shows as copy in organic listings; website owners do not.
- There has yet to be any specific direction from FDA on metadata and no rules requiring their adherence to the “reminder” format in place for paid search ads – specifically:
- In sending 14 enforcement letters pertaining to paid search (SEM) campaigns to pharmaceutical companies in 2009, FDA weighed in forcefully against the practice of limited space ad copy making brand claims without meeting all of the requirements for a full product promotion. The absence of any corresponding direction explicitly addressing metadata and organic listings in 2009 and for the 7 years since suggests that meta data is seen in a different light.
- The industry has taken the cue, with many pharma brands using meta titles and descriptions that include brand names and claims that correspond with the contents of their respective web pages, much like the algorithms themselves might generate in the absence of site-provided metadata.
- In the absence of formal rules preventing their use, fully transparent meta data offers search engines more accurate and transparent descriptions of a drug than can be deduced by an algorithm. The result is an arguably superior user experience for patients and HCPs, as metadata can more accurately represent page content. The core goal of search engines is to communicate to the searcher the contents of the indexed pages, and titles and descriptions help the engines maximize accuracy to users when includes condition information. As we argued in our previous post in defense of controversial paid search keywords, given the abundance of incomplete and inaccurate information about drugs online, it serves the public interest for consumers to have unfettered visibility to the drug information provided by an FDA-regulated information source. Uncensored meta data makes that possible.
As implied above, a third option that pharma website owners have is to omit title and/or description tags altogether, leaving search engines to select content from the indexed webpage that it deems most relevant for inclusion in the organic result. We do not recommend this option as it introduces a greater degree of uncertainty to the process, leaving it entirely to the engines as to how to describe the page, site and/or product.
When a brand chooses not to restrict its meta tag content to conform with the “reminder ad” format, we recommend applying best practice guidelines:
- In meta descriptions, include language encouraging searchers to read the brand’s important safety/risk info, and ensure that the full ISI is included on the linked page
- Use copy consistent with drug’s approved indication and avoid being misleading
- Include brand name and generic name
- Indicate the intended target audience (Patient/HCP/Caregivers)
Branded Website Meta tags – The Good, the Conservative and the Ugly
Example condition: Pediatric Relapsing MS
|
Homepage Meta Title and Description Tag |
Thoughts |
|
| Good | Brand® (generic name) for Pediatric Relapsing MS
Learn about Brand as a treatment option for pediatric relapsing MS including risks and side effects information for patients & caregivers. |
|
| Conservative | Brand® (generic name) – Official HCP Site
Learn about Brand, side effects and financial assistance info for your patients. |
|
| Ugly | Brand® (generic name) for MS Cure
Learn about the best treatment for MS cure. |
|
This article has been reposted with permission from Convergence Point Media. Click here to read the original posting.