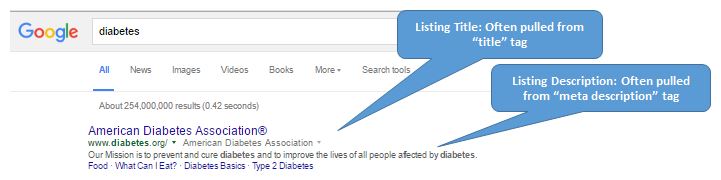
On February 17th, the Movember Foundation went beyond the moustache by expanding their promotional efforts through PatientPoint. With prostate cancer now the second most common form of cancer seen in men in the U.S., the two organizations teamed up to spread awareness and inspire healthy action between men and their healthcare professionals. According to Paul Villanti, the executive director of the Movember Foundation, “too many men don’t talk about their health, don’t take action, and as a result, they die too young from diseases that are often treatable”. PatientPoint programs impact half a billion patient and caregiver visits each year –this kind of reach will be huge for Movember Foundation and help them push towards their goal: to stop men dying too young.
On February 17th, the Movember Foundation went beyond the moustache by expanding their promotional efforts through PatientPoint. With prostate cancer now the second most common form of cancer seen in men in the U.S., the two organizations teamed up to spread awareness and inspire healthy action between men and their healthcare professionals. According to Paul Villanti, the executive director of the Movember Foundation, “too many men don’t talk about their health, don’t take action, and as a result, they die too young from diseases that are often treatable”. PatientPoint programs impact half a billion patient and caregiver visits each year –this kind of reach will be huge for Movember Foundation and help them push towards their goal: to stop men dying too young.
To learn more about Movember, click here, or to read the news release, click here.