Here’s the big question: How does an industry with regulatory constraints around how it communicates with the public successfully engage on social media through robust, timely, and helpful interactions?
There’s not a simple answer, but creating clear and consistent “rules of engagement” can make for a good first step forward.
As a digital and social media strategist for C3i Healthcare Connections, I help pharma clients build out their social presence and extract meaningful information that can be gleaned from social media. While ongoing monitoring of conversations is a key component of any pharmaceutical brand’s social strategy (see my previous article here), companies need to be well-equipped to participate in those conversations, too. Five broad steps can help you get started.
- Develop engagement strategies
Take the time to outline your plan of attack, along with the ways your team can apply these principles to their everyday interactions. Doing so will benefit everyone involved by making operations more efficient and streamlined, your customers more satisfied, and your brand more favorable.
 Start by assessing the engagement opportunities available to non-regulated industries and rule out those activities which regulations prohibit, such as providing off-label information, soliciting or prompting users to share content that might lead to off-label questions, and recommending or directly promoting the use of a product to a user. After implementing compliance safeguards for handling Adverse Events (AEs), Product Quality Complaints (PQCs), and Privacy Violations (see this article), you can begin to develop your own engagement strategies and practices.
Start by assessing the engagement opportunities available to non-regulated industries and rule out those activities which regulations prohibit, such as providing off-label information, soliciting or prompting users to share content that might lead to off-label questions, and recommending or directly promoting the use of a product to a user. After implementing compliance safeguards for handling Adverse Events (AEs), Product Quality Complaints (PQCs), and Privacy Violations (see this article), you can begin to develop your own engagement strategies and practices.
As part of this process, you should:
- Work with key stakeholders — including marketing and branding, public relations, medical information, and pharmacovigilance — to identify the objectives of engagement
- Be prepared to identify and report any AEs and PQCs on owned properties (e.g., branded or unbranded Facebook Pages) and any properties over which the pharma company has control or influence
- Evaluate the current social media space and your role in it
- Develop workflows and escalation guidelines, perhaps considering third-party technologies that help streamline workflows and support operational evaluation
- Establish community guidelines — besides guidelines for posting and commenting, this may include a statement that explains the purpose of the property, links, and contact information
- Be consistent … but human and flexible, too
After you’ve decided to move beyond monitoring and begin engaging on social media, many companies often start with a simple first-step strategy of responding to AEs and PQCs with a “contact-us reply.” For example: “Hi Sarah. Thank you for bringing this to our attention. We take product safety seriously and are interested in learning more about your experience. Please call us at 800-555-5555.”
A standardized “contact-us reply” can sometimes feel robotic. While remaining consistent is important, it’s equally necessary for brands to consider how to bring the human element to their interactions. Beyond AEs/PQCs replies, teams can add a personal touch by taking the initiative to respond to on-label inquiries or consumer sharing of experiences: “Thank you for sharing, John. We’re glad that you’re taking steps to manage your health.”
Being more human means listening carefully to what is being asked and acknowledging what has been stated. Text can be difficult to interpret sometimes, but you can take cues from emojis, emoticons, and images. While providing accurate on-label information is critical, so too is the emotional tone of an interaction, especially in the sensitive area of health.
- Establish KPIs
If something can be observed, it can be measured. Two kinds of key performance indicators (KPIs) help assess the performance of your interactions and resource needs of your initiatives. They are productivity KPIs and volumetric KPIs.
Productivity KPIs include metrics such as:
- First Response Time — the time between the consumer’s first contact and the company’s first response for all engagements over a period of time
- Response Time — the total time from the consumer’s first contact and the company’s last response
- Handle Time — the time from the consumer’s first engagement and the completion of all tasks required to process a case
- Resolution Rate — the ratio of the number of resolved posts to the number of those that needed resolution
In today’s world, consumers expect swift responses. In an ideal world with infinite resources, response times on social media could be less than a minute. In reality, however, resources can limit optimal response times. Establish your initial KPI standards based on the number of people on your staff, hours of labor, and average number of posts per hour they can handle. Often vendors can assist in the heavy load of supporting customer care in cooperation with your company.
Volumetric KPIs might include the total of all posts in a given period for a given property (e.g., total Facebook posts in May), or the volume of posts for which the company responded, which can be further organized by type of post (AEs, PQCs, product inquiries, etc.). Third-party technologies can assist with these KPI measurements, although some are better suited for monitoring and reporting, while others are built to support consumer care and interaction. Your technology selection depends on the objectives of your engagement strategy.
Besides supporting the initial stages of your engagement efforts, tracking KPIs after they have been established helps to identify areas of improvement and opportunities within your operations.
- Prepare for the unexpected
No matter how refined your social strategy, there are always surprises. While you can’t control unexpected events, you can prepare for them.
Before launching your social media initiative, carefully document the process for escalating an issue depending on the situation presented. Establish escalation criteria and communication protocols to avoid last-minute panic. Be transparent, and continually monitor the situation until it resolves.
Keep in mind that a response isn’t necessarily an answer. Make sure your teams can distinguish legitimate consumer concerns from spam content. If a consumer posts an inquiry and an immediate answer is not available, it’s OK to acknowledge the question and inform the consumer that he or she will receive a follow-up response. Suggesting a private message can be another effective way to handle, or public responses that benefit the community.
- Evaluate performance, apply insights, and adjust practices
In social media, as in any initiative, there is always room for improvement, refinement, and course-correction. For example, if average response and handle time goals are not being met, is it due to a lack of staffing (a volume issue), or a need to coach your representatives? On the flip side, if response times are quicker than anticipated, are there other activities that can be added to the initiative, such as improving the quality of responses?
A big advantage social media has over traditional media is the ability to more immediately measure and evaluate the performance of content. As experience is gained and insights are gleaned, proceed to evolve from a passive/reactive model to an active approach that seeks out opportunities for engagement.
Consumers are eager to receive information and support from all parties in the healthcare system. Those pharma companies or brands that have established the foundations for social media processes and, ultimately, build up to higher tiers of engagement, not only have a greater opportunity to meet or exceed patient expectations — they’re also able to earn long-term trust and favorability among patients.


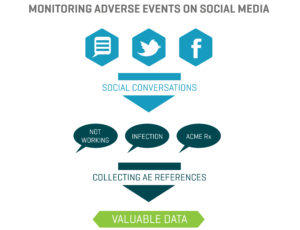
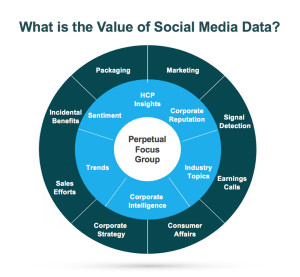 In the healthcare industry, patients and healthcare providers (HCPs) are looking to social media for information and support regarding their health and the health of their patients. They serve as a “perpetual focus group,” whose conversations taking place on social media provide brands with the opportunity to listen. Patients share their opinions, ask questions about diseases and treatment options, and directly or incidentally report adverse events. Meanwhile, HCPs voice opinions on the healthcare industry, provide thought leadership on a wide range of topics such as participatory medicine, reimbursement, and medical education. In a recent survey, more than 40 percent of respondents reported that information found via social media would affect the way they coped with a chronic condition or their approach to diet and exercise; 34 percent said it would affect taking certain medication.1 This demonstrates to companies that patients are directly impacted by what they view on social media and in order for brands to leverage that data radiating out of all the social conversations, several factors need to be in place.
In the healthcare industry, patients and healthcare providers (HCPs) are looking to social media for information and support regarding their health and the health of their patients. They serve as a “perpetual focus group,” whose conversations taking place on social media provide brands with the opportunity to listen. Patients share their opinions, ask questions about diseases and treatment options, and directly or incidentally report adverse events. Meanwhile, HCPs voice opinions on the healthcare industry, provide thought leadership on a wide range of topics such as participatory medicine, reimbursement, and medical education. In a recent survey, more than 40 percent of respondents reported that information found via social media would affect the way they coped with a chronic condition or their approach to diet and exercise; 34 percent said it would affect taking certain medication.1 This demonstrates to companies that patients are directly impacted by what they view on social media and in order for brands to leverage that data radiating out of all the social conversations, several factors need to be in place.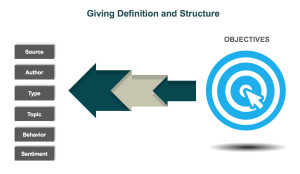 d is looking to identify who is talking about a product, the kinds of content they are posting, and the topics within their content, a structure that would classify posts into categories such as Consumer, Branded Product, and Medical Inquiry could be utilized. As more and more posts are tagged, patterns, trends, and themes can be identified.
d is looking to identify who is talking about a product, the kinds of content they are posting, and the topics within their content, a structure that would classify posts into categories such as Consumer, Branded Product, and Medical Inquiry could be utilized. As more and more posts are tagged, patterns, trends, and themes can be identified.