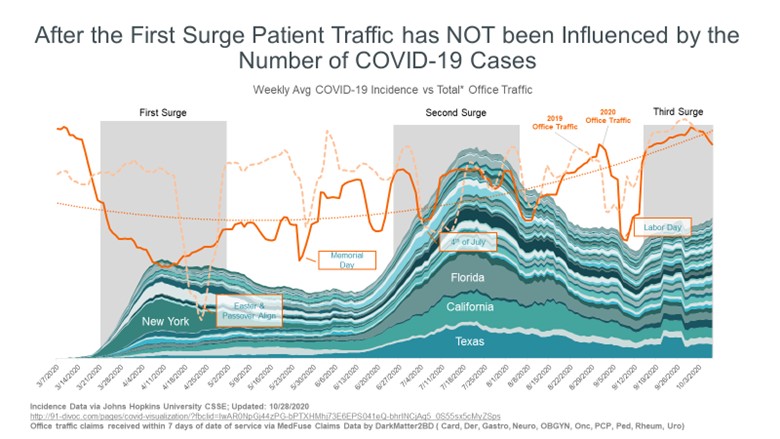
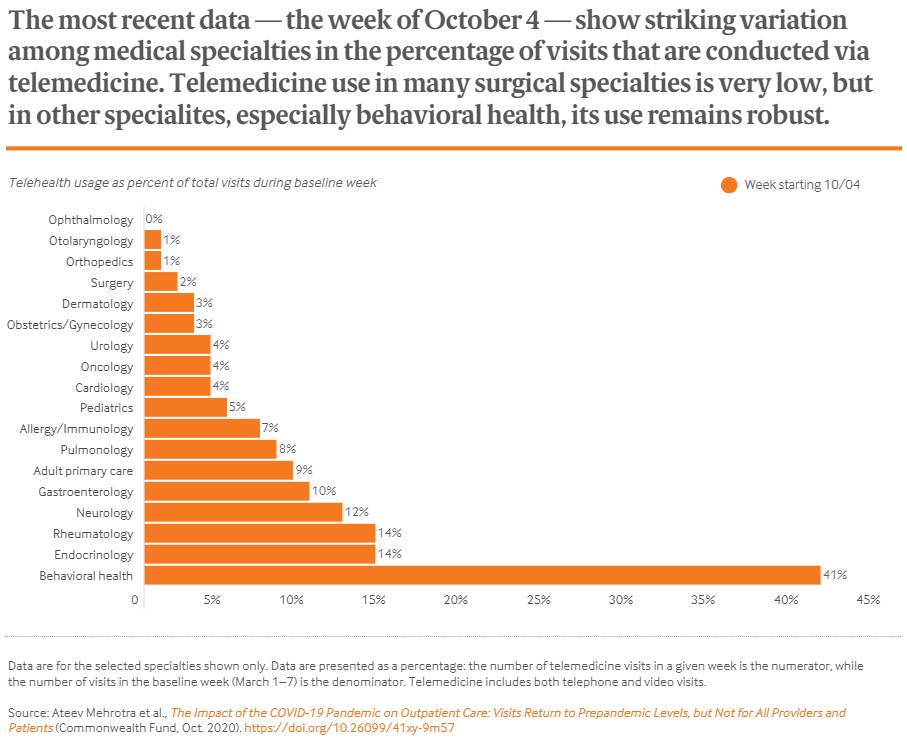
In honor of American Heart Month in February, PatientPoint and the American Heart Association have partnered to highlight stories from heart attack and stroke survivors from the 2021 Class of Go Red for Women® Real Women campaign. These stories will be shared on PatientPoint’s digital waiting room screens and exam room touchscreens throughout the US over the course of the rest of the year.
According to the news release, among the Real Women featured are:
- Dani Aylsworth: A 33-year-old Army combat veteran who battled PTSD, addiction, and heart failure.
- Megan Corbin: A 30-year-old professional dancer who had a heart attack despite being in top form.
- Melissa Sloan-Williams: A 39-year-old registered medical assistant who developed Type 2 diabetes and had a heart attack in her early 30s.
- Steffany Quintana: A 26-year-old who lost the ability to talk, swallow food, and walk unassisted after her stroke last year.
Go Red for Women, which is sponsored by CVS Health, is an initiative from the American Heart Association to raise awareness and end heart disease and stroke in women. As noted in the Real Stories video, one in three women will die from cardiovascular disease annually, making it the leading killer of women in the US. The news release also cited how “research shows heart attacks are on the rise in younger women and new data suggests younger generations of women, Gen Z and Millennials, along with Black and Hispanic women, are less likely to be aware of their greatest health threat, including knowing the warning signs of heart attacks and strokes.”
The Real Stories at the point of care serves to educate women so they understand the signs and symptoms (which are often different symptoms than what men experience) and to inspire them to make heart health a top priority in conjunction with their overall health management.